Ionization Unveiled
An in-depth exploration of the fundamental process by which atoms and molecules gain or lose electrons, shaping the physical world.
What is Ionization? 👇 Explore Applications 🔬Dive in with Flashcard Learning!
🎮 Play the Wiki2Web Clarity Challenge Game🎮
What is Ionization?
Acquiring Charge
Ionization is the physical process through which an atom or molecule acquires a net electrical charge. This transformation occurs when an atom or molecule either gains or loses one or more electrons, fundamentally altering its electrical state.[1] The resulting electrically charged atom or molecule is known as an ion.[1] This process can be initiated by various interactions, including collisions with subatomic particles, other atoms, or electromagnetic radiation.[1][2]
Mechanisms of Ionization
Ionization can result from several mechanisms:
- Collisional Ionization: Occurs when an atom or molecule collides with other particles, such as electrons, positrons, protons, or other ions, transferring sufficient energy to eject an electron.
- Photoionization: Happens when an atom or molecule absorbs a photon of electromagnetic radiation with enough energy to eject an electron.
- Field Ionization: Takes place when a strong external electric field distorts the atom's or molecule's potential barrier, allowing an electron to escape via quantum tunneling.
- Thermal Ionization: Occurs at high temperatures, where thermal energy is sufficient to overcome the binding energy of electrons.
These processes are critical in understanding phenomena ranging from atmospheric physics to plasma generation.
Ionization in Nature
Ionization is a ubiquitous phenomenon in the universe. A striking example is the formation of auroras. The solar wind, composed of charged particles, interacts with Earth's magnetosphere, altering the movement of charged particles in the upper atmosphere. This ionization causes these particles to emit light, creating the spectacular displays near the polar regions.[1]
Key Applications
Lighting and Detection
Ionization is fundamental to the operation of many technologies:
- Gas Discharge Lamps: Devices like fluorescent lamps and neon signs rely on the ionization of gases within a tube to produce light.
- Radiation Detectors: Instruments such as Geiger-Müller counters and ionization chambers detect ionizing radiation by measuring the electrical current produced when radiation ionizes the gas within the detector.
Scientific and Medical Uses
In scientific research and medical applications, ionization plays a vital role:
- Mass Spectrometry: This analytical technique uses ionization to convert molecules into ions, allowing their mass-to-charge ratio to be measured for identification and quantification.
- Radiation Therapy: High-energy radiation used in cancer treatment works by ionizing cells, damaging their DNA and leading to cell death.
Generating Ions
Negative Ion Formation
Negative ions are formed when an atom or molecule captures a free electron. This process, known as electron capture ionization, typically occurs when a free electron collides with a neutral atom or molecule and becomes bound within its electric potential well.[14]
Positive Ion Formation
Positive ions are created when an atom or molecule loses an electron. This requires imparting a minimum amount of energy, known as the ionization energy, to a bound electron. This energy can be supplied through collisions with charged particles (like electrons or other ions) or via absorption of photons.[15]
The Townsend Discharge
A classic example of positive ion and electron generation is the Townsend discharge. In a gas subjected to a sufficiently strong electric field, an initial ionization event can trigger a cascade. The liberated electron accelerates towards the anode, gaining enough energy to ionize other gas molecules upon collision. This process, known as an electron avalanche, sustains a continuous electrical current.[17] The efficiency of this process is quantified by the ionization efficiency, which is the ratio of ions formed to the number of incident electrons or photons.[18][19]
Ionization Energy Trends
Periodic Behavior
The ionization energy of atoms, the minimum energy required to remove an electron, exhibits predictable trends across the periodic table. These trends are invaluable for understanding the arrangement of electrons in atomic orbitals without delving into complex quantum mechanical wave functions.[10] For instance, the sharp decrease in ionization energy after noble gases signifies the start of a new electron shell in alkali metals.
Atomic Structure Insights
Analyzing ionization energies provides direct insight into atomic structure. Peaks in ionization energy plots correspond to the filling of sub-shells (s, p, d, f), reflecting the stability associated with these configurations. This relationship is a cornerstone of chemical periodicity.

Quantum Mechanical Descriptions
Interaction with Fields
The ionization of atoms and molecules by intense laser pulses or other charged particles is a complex quantum mechanical process. Calculating the ionization rate requires sophisticated methods, including perturbative and non-perturbative approaches. Techniques like time-dependent coupled-channel methods or solving the Schrödinger equation numerically on a lattice are employed to model these interactions accurately.[22][23][24][25][26][27]
Perturbative vs. Non-Perturbative
When the laser intensity is extremely high, simplifying approximations can lead to analytic solutions for the ionization rate. However, for more general cases, numerical solutions are often necessary. The Keldysh parameter () is a key parameter that distinguishes between multiphoton ionization (MPI) and tunnel ionization regimes.[28]
The Keldysh Model
The Keldysh model provides a framework for understanding MPI, treating it as a transition to Volkov states. However, it neglects the influence of the Coulomb interaction on the final electron state. More refined models, like the PPT (Perelomov et al.) model, incorporate these Coulomb effects as first-order corrections, offering improved accuracy, particularly in intermediate regimes of the Keldysh parameter.[30][31]
Tunnel Ionization
Quantum Tunneling
Tunnel ionization is a quantum mechanical phenomenon where an electron escapes an atom or molecule not by overcoming a potential barrier classically, but by tunneling *through* it. This probability is exponentially dependent on the barrier's width. The thinner the barrier, the higher the probability of tunneling. This process is particularly relevant when atoms interact with intense laser fields.
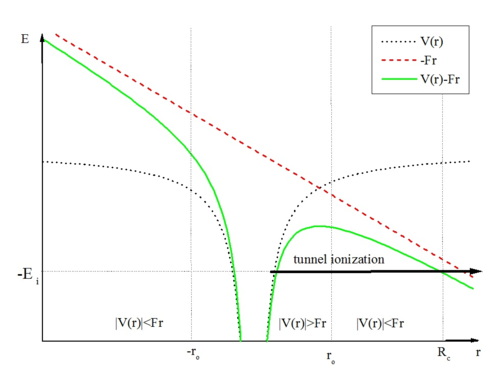
Quasi-Static Tunnel Ionization (QST)
The quasi-static tunneling (QST) model, derived as a limit of the PPT model, provides a robust prediction for ionization rates. It simplifies the complex quantum dynamics by considering the laser field as quasi-static relative to the electron's motion. The ADK (Ammosov-Delone-Krainov) model is a prominent example of QST theory.[33][34]
Strong Field Approximation
The Strong Field Approximation (SFA) offers alternative methods for calculating ionization rates, often emphasizing the particle nature of light. Krainov's model, for instance, utilizes SFA principles and incorporates corrections like the Coulomb potential's influence on the electron's final state, aligning well with experimental observations.[35]
The Kramers-Henneberger Frame
A Moving Reference Frame
The Kramers-Henneberger (KH) frame is a non-inertial reference frame specifically designed for analyzing atomic and molecular interactions with intense laser fields. In this frame, the classical motion of an electron under the laser's influence is effectively frozen, simplifying the description of the system's dynamics.[58][59]
Simplified Hamiltonian
By transforming the laboratory-frame Hamiltonian, the KH frame yields a Hamiltonian that includes the original potential centered on the oscillating position of the electron (). This formulation is particularly useful for applying high-frequency Floquet theory, simplifying the analysis of phenomena like atomic stabilization.[60][61]
Applications
The KH frame has been instrumental in theoretical studies of high-harmonic generation and atomic stabilization, providing a framework to understand complex interactions between matter and intense laser fields.[62]
Distinguishing Ionization from Dissociation
Charge vs. Structure
It is crucial to distinguish ionization from dissociation. Dissociation involves the breaking of chemical bonds within a molecule, potentially forming smaller neutral or charged fragments, but not necessarily involving a net gain or loss of electrons by the original molecular entity. For instance, sugar dissolving in water involves dissociation but not ionization, as the sugar molecules break apart but remain neutral.[63]
Ionization of Ionic Compounds
In contrast, the dissociation of sodium chloride (table salt) in water illustrates ionization. Here, the salt crystal lattice breaks down, releasing pre-existing sodium (Na+) and chloride (Cl-) ions. While these ions become solvated by water molecules, the process itself does not involve the transfer or displacement of electrons between the sodium and chlorine species; they were already ionic.
Phase Transitions
States of Matter
Ionization represents a transition to the plasma state, distinct from other phase changes like melting or vaporization. The table below illustrates the common phase transitions between solid, liquid, gas, and plasma.
To From
|
Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Deposition | Condensation | Ionization | |
| Plasma | Recombination |
Related Concepts
Further Exploration
To deepen your understanding of ionization, consider exploring these related topics:
- Above Threshold Ionization
- Double Ionization
- Chemical Ionization
- Electron Ionization
- Ionization Chamber
- Ion Source
- Photoionization
- Thermal Ionization
- Townsend Avalanche
References
Source Material
- Machacek, J.R.; McEachran, R.P.; Stauffer, A.D. (2023). "Positron Collisions". Springer Handbook of Atomic, Molecular, and Optical Physics. Springer Handbooks. Springer. doi:10.1007/978-3-030-73893-8_51. ISBN 978-3-030-73892-1.
- Kirchner, Tom; Knudsen, Helge (2011). "Current status of antiproton impact ionization of atoms and molecules: theoretical and experimental perspectives". Journal of Physics B: Atomic, Molecular and Optical Physics. 44 (12) 122001. Bibcode:2011JPhB...44l2001K. doi:10.1088/0953-4075/44/12/122001.
- Brandsen, B.H. (1970). Atomic Collision Theory. Benjamin. ISBN 978-0-8053-1180-8.
- Stolterfoht, N; DuBois, R.D.; Rivarola, R.D. (1997). Electron Emission in Heavy Ion-Atom Collisions. Springer-Verlag. ISBN 978-3-642-08322-8.
- McGuire, J.H. (1997). Electron correlation dynamics in atomic collisions. Cambridge University Press. ISBN 978-0-521-48020-8.
- Eichler, J. (2005). Lectures on Ion-Atom Collisions: From Nonrelativistic to Relativistic Velocities. Elsevier. ISBN 978-0-444-52047-0.
- Bransden, B.H.; McDowell, M.R.C. (1992). Charge Exchange and the Theory of Ion-Atom Collisions. Clarendon Press; Oxford University Press. ISBN 978-0-19-852020-7.
- Janev, R.K.; Presnyakov, L.P.; Shevelko, V.P. (1985). Physics of Highly Charged Ions. Springer. ISBN 978-3-642-69197-3.
- Schulz, Michael (2019). Schulz, Michael (ed.). Ion-Atom Collisions The Few-Body Problem in Dynamic Systems. De Gruyter. doi:10.1515/9783110580297. ISBN 978-3-11-057942-0.
- D., Belkic (2009). Quantum Theory of High-Energy Ion-Atom Collisions. CRC Press. ISBN 978-1-58488-728-7.
- Schmelcher, P.; Schweitzer, W. (2002). Atoms and Molecules in Strong External Fields. Kulver Academic Publishers. ISBN 0-306-45811-X.
- Waring, M. S.; Siegel, J. A. (August 2011). "The effect of an ion generator on indoor air quality in a residential room: Effect of an ion generator on indoor air in a room". Indoor Air. 21 (4): 267–276. doi:10.1111/j.1600-0668.2010.00696.x. PMID 21118308.
- University, Colorado State. "Study uncovers safety concerns with ionic air purifiers". phys.org. Retrieved 2023-06-28.
- Andersen, T (2004). "Atomic negative ions: structure, dynamics and collisions". Physics Reports. 394 (4–5): 157–313. Bibcode:2004PhR...394..157A. doi:10.1016/j.physrep.2004.01.001.
- Schulz, Michael (2003). "Three-Dimensional Imaging of Atomic Four-Body Processes". Nature. 422 (6927): 48–51. Bibcode:2003Natur.422...48S. doi:10.1038/nature01415. hdl:11858/00-001M-0000-0011-8F36-A. PMID 12621427. S2CID 4422064.
- IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "adiabatic ionization". doi:10.1351/goldbook.A00143
- Glenn F Knoll. Radiation Detection and Measurement, third edition 2000. John Wiley and sons, ISBN 0-471-07338-5
- Todd, J. F. J. (1991). "Recommendations for Nomenclature and Symbolism for Mass Spectroscopy (including an appendix of terms used in vacuum technology)(IUPAC Recommendations 1991)". Pure Appl. Chem. 63 (10): 1541–1566. doi:10.1351/pac199163101541.
- IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "ionization efficiency". doi:10.1351/goldbook.I03196
- Abrines, R.; Percival, I.C. (1966). "Classical theory of charge transfer and ionization of hydrogen atoms by protons". Proceedings of the Physical Society. 88 (4): 861–872. Bibcode:1966PPS....88..861A. doi:10.1088/0370-1328/88/4/306.
- Schulz, Michael (2003). "Three-Dimensional Imaging of Atomic Four-Body Processes". Nature. 422 (6927): 48–51. Bibcode:2003Natur.422...48S. doi:10.1038/nature01415. hdl:11858/00-001M-0000-0011-8F36-A. PMID 12621427. S2CID 4422064.
- Krainov, V. P. (1997). "Atomic ionization by intense laser fields". Physics of Atomic Nuclei. 60 (10): 1730–1734.
- Faisal, F. H. M. (1973). "Multiple photon absorption by an atom". Journal of Physics B: Atomic and Molecular Physics. 6 (3): 479. doi:10.1088/0022-3700/6/3/010.
- Reiss, H. R. (1980). "Analytic theory of the response of atoms to intense laser fields". Physical Review A. 22 (2): 629. doi:10.1103/PhysRevA.22.629.
- Perelomov, A. M.; Popov, V. S.; Terent'ev, M. V. (1966). "Ionization of hydrogen-like atoms in a variable field". Theoretical and Mathematical Physics. 4 (1): 52–61. doi:10.1007/BF01037303.
- Larochelle, S.; et al. (1995). "Ionization of rare-gas atoms by a Ti:sapphire laser". Physical Review A. 52 (5): 3782. doi:10.1103/PhysRevA.52.3782.
- Shakeshaft, R.; Spruch, L. (1986). "WKB approximation for the calculation of ionization rates". Reviews of Modern Physics. 58 (3): 745. doi:10.1103/RevModPhys.58.745.
- Keldysh, L. V. (1965). "Ionization in the field of a strong electromagnetic wave". Soviet Physics JETP. 20: 1307.
- Volkov, D. M. (1935). "Radiation of electron in the field of electromagnetic wave". Zeitschrift für Physik. 94 (4-5): 250–260. doi:10.1007/BF01331074.
- Perelomov, A. M.; Popov, V. S.; Terent'ev, M. V. (1966). "Ionization of hydrogen-like atoms in a variable field". Theoretical and Mathematical Physics. 4 (1): 52–61. doi:10.1007/BF01037303.
- Perelomov, A. M.; Popov, V. S. (1967). "Ionization of atoms in a field of intense electromagnetic radiation". Soviet Physics JETP. 24: 50.
- Larochelle, S.; et al. (1995). "Ionization of rare-gas atoms by a Ti:sapphire laser". Physical Review A. 52 (5): 3782. doi:10.1103/PhysRevA.52.3782.
- Ammosov, V. V.; Delone, N. B.; Krainov, V. P. (1986). "Polarization of atomic residues after ionization by a strong electromagnetic field". Soviet Physics JETP. 64 (6): 1191.
- Sharifi, M.; Talebpour, A. (2010). "Tunnel ionization of atoms in intense laser fields". Journal of Optics. 12 (11): 115501. doi:10.1088/2040-8978/12/11/115501.
- Krainov, V. P. (1997). "Atomic ionization by intense laser fields". Physics of Atomic Nuclei. 60 (10): 1730–1734.
- Faisal, F. H. M. (1973). "Multiple photon absorption by an atom". Journal of Physics B: Atomic and Molecular Physics. 6 (3): 479. doi:10.1088/0022-3700/6/3/010.
- Reiss, H. R. (1980). "Analytic theory of the response of atoms to intense laser fields". Physical Review A. 22 (2): 629. doi:10.1103/PhysRevA.22.629.
- Story, J. G.; Duncan, C. B. (1994). "Population trapping in multiphoton ionization". Physical Review A. 49 (3): 1978. doi:10.1103/PhysRevA.49.1978.
- de Boer, M. J.; Muller, H. G. (1992). "Population trapping in multiphoton ionization". Physical Review A. 45 (1): 110. doi:10.1103/PhysRevA.45.110.
- Story, J. G.; Duncan, C. B. (1994). "Population trapping in multiphoton ionization". Physical Review A. 49 (3): 1978. doi:10.1103/PhysRevA.49.1978.
- Talebpour, A.; et al. (1996). "Structures in the ionization yields of rare gases by short laser pulses". Physical Review A. 54 (2): 1602. doi:10.1103/PhysRevA.54.1602.
- Morishita, T.; Lin, C. D. (2013). "Population trapping in strong laser fields". Journal of Physics B: Atomic, Molecular and Optical Physics. 46 (15): 155001. doi:10.1088/0953-4075/46/15/155001.
- L'Huillier, A.; et al. (1983). "High-order harmonic generation in atomic gases". Physical Review Letters. 51 (19): 1715. doi:10.1103/PhysRevLett.51.1715.
- Augst, S.; et al. (1989). "Ionization of atoms by intense laser fields". Physical Review Letters. 63 (20): 2212. doi:10.1103/PhysRevLett.63.2212.
- Walker, B.; et al. (1994). "Space-charge effects in strong-field ionization". Physical Review Letters. 73 (10): 1368. doi:10.1103/PhysRevLett.73.1368.
- Fittinghoff, D. N.; et al. (1992). "Atomic ionization by intense laser fields: Shake-off model". Physical Review A. 45 (3): 1978. doi:10.1103/PhysRevA.45.1978.
- Kuchiev, M. Y. (1997). "Non-sequential double ionization of atoms in intense laser fields". Journal of Physics B: Atomic, Molecular and Optical Physics. 30 (1): L7. doi:10.1088/0953-4075/30/1/002.
- Schafer, W. T.; et al. (1997). "Theory of multiphoton ionization of atoms in intense laser fields". Physical Review A. 55 (6): 4473. doi:10.1103/PhysRevA.55.4473.
- Corkum, P. B. (1993). "High-intensity phenomena". Phys. Rev. Lett. 71 (13): 1994. doi:10.1103/PhysRevLett.71.1994.
- Becker, W.; Faisal, F. H. M. (1996). "Theory of non-sequential double ionization of atoms in intense laser fields". Physical Review A. 54 (4): 3248. doi:10.1103/PhysRevA.54.3248.
- Faisal, F. H. M.; Becker, W. (1997). "Theory of non-sequential double ionization of atoms in intense laser fields". Physical Review A. 55 (6): 4473. doi:10.1103/PhysRevA.55.4473.
- Walker, B.; et al. (1994). "Space-charge effects in strong-field ionization". Physical Review Letters. 73 (10): 1368. doi:10.1103/PhysRevLett.73.1368.
- Becker, W.; Faisal, F. H. M. (1996). "Theory of non-sequential double ionization of atoms in intense laser fields". Physical Review A. 54 (4): 3248. doi:10.1103/PhysRevA.54.3248.
- Agostini, P.; et al. (1993). "Multiphoton ionization of inner-valence electrons and fragmentation of polyatomic molecules". Journal of Physics B: Atomic, Molecular and Optical Physics. 26 (1): L1. doi:10.1088/0953-4075/26/1/002.
- Agostini, P.; et al. (1994). "Multiphoton ionization of molecules". Journal of Physics B: Atomic, Molecular and Optical Physics. 27 (1): 1. doi:10.1088/0953-4075/27/1/001.
- Peng, L.; Puskas, J. (2012). "Short pulse induced molecular fragmentation for mass spectroscopy". Rapid Communications in Mass Spectrometry. 26 (1): 1. doi:10.1002/rcm.5270.
- Peng, L.; Puskas, J. (2012). "Short pulse induced molecular fragmentation for mass spectroscopy". Rapid Communications in Mass Spectrometry. 26 (1): 1. doi:10.1002/rcm.5270.
- Kramers, H. A. (1926). "Quantentheorie und elfdimensionale Raumzeit". Zeitschrift für Physik. 39 (10): 828–840. doi:10.1007/BF01397274.
- Henneberger, W. C. (1968). "Electric-field-induced shifts of energy levels". Physical Review Letters. 21 (17): 1155. doi:10.1103/PhysRevLett.21.1155.
- Reiss, H. R. (1990). "Atomic physics in strong fields". Progress in Quantum Electronics. 14 (1): 1–70. doi:10.1016/0079-6727(90)90001-X.
- Faisal, F. H. M. (1990). "Atomic stabilization in intense laser fields". Journal of Physics B: Atomic, Molecular and Optical Physics. 23 (1): L1. doi:10.1088/0953-4075/23/1/001.
- Gong, Z.; et al. (2011). "High-harmonic generation from a metal surface in a powerful laser field". Physical Review A. 84 (4): 043413. doi:10.1103/PhysRevA.84.043413.
- Agostini, P.; et al. (1993). "Multiphoton ionization of inner-valence electrons and fragmentation of polyatomic molecules". Journal of Physics B: Atomic, Molecular and Optical Physics. 26 (1): L1. doi:10.1088/0953-4075/26/1/002.
Teacher's Corner
Edit and Print this course in the Wiki2Web Teacher Studio

Click here to open the "Ionization" Wiki2Web Studio curriculum kit
Use the free Wiki2web Studio to generate printable flashcards, worksheets, exams, and export your materials as a web page or an interactive game.
True or False?
Test Your Knowledge!
Gamer's Corner
Are you ready for the Wiki2Web Clarity Challenge?

Unlock the mystery image and prove your knowledge by earning trophies. This simple game is addictively fun and is a great way to learn!
Play now
References
References
- Gavrila, Mihai. "Atomic structure and decay in high-frequency fields." Atoms in Intense Laser Fields, edited by Mihai Gavrila, Academic Press, Inc, 1992, pp. 435-508.
Feedback & Support
To report an issue with this page, or to find out ways to support the mission, please click here.
Disclaimer
Important Notice
This page was generated by an Artificial Intelligence and is intended for informational and educational purposes only. The content is based on a snapshot of publicly available data from Wikipedia and may not be entirely accurate, complete, or up-to-date.
This is not professional advice. The information provided on this website is not a substitute for professional scientific consultation, theoretical physics analysis, or experimental design advice. Always refer to official documentation and consult with qualified professionals for specific needs.
The creators of this page are not responsible for any errors or omissions, or for any actions taken based on the information provided herein.

